
Public domain image
Over recent years, the importance of drug approval and patent linkage systems—particularly the latter—have frequently emerged in the course of the negotiation of international free trade agreements (FTAs). Countries entering into FTAs with the USA, including Australia, Canada, Singapore and South Korea, have created patent linkage systems, or similar systems, to strengthen intellectual property protection for pharmaceuticals. After Taiwan announced that it would amend the Pharmaceutical Affairs Act and Patent Act and establish patent linkage in the quest for closer trade relations with the USA, the generic drug industry in Taiwan has expressed concerns over the potential adverse effects with regard to access to less expensive drugs and the impact this may have on public health.
This article reviews briefly the main characteristics of patent linkage, its implementation in the USA and other countries, and the inefficiencies of the current IPR-declaration mechanism in Taiwan. On the basis of the growing numbers of pharmaceutical patents filed by domestic inventors, the author asserts that early resolution mechanisms for avoiding disputes over pharmaceutical patents are in compliance with knowledge-based economic development and the goal of encouraging innovation and increasing the competitiveness of Taiwan’s domestic industry.
Background Information on Patent Linkage in the USA
It has been well-documented that the USA was the first country to establish a complex patent linkage system, introduced by the Drug Price Competition and Patent Term Restoration Act of 1984 (Pub. L. No. 98-417)—commonly referred to as the Hatch-Waxman Act. On one hand the Hatch-Waxman Act enhanced intellectual property protection, enabling innovative branded drugmakers which had filed New Drug Applications (NDA) to benefit from the mechanisms of patent term extension and data exclusivity; while, on the other hand, it accelerated the introduction of generic drugs on to the market and allowed generic drugmakers to take advantage of the simplified Abbreviated New Drug Application (ANDA) approval process and the statutory research exemption (Bolar provision).
Table 1: Main mechanisms introduced by the Hatch-Waxman Act
In Favor of Branded Drugmakers (NDA Holders) |
In Favor of Generic Drugmakers (ANDA Holders) |
patent term extension |
ANDA process |
data exclusivity |
statutory research exemption |
In addition to the above-mentioned mechanisms, the Hatch-Waxman Act also created patent linkage, aimed at establishing the statutory framework for a competitive environment in the pharmaceutical industry. Patent linkage is an early resolution mechanism for avoiding disputes over pharmaceutical patents. It prevents the marketing of generic drugs from infringing patents on the drugs they reference. To achieve this policy goal, four main mechanisms have been put in place. These are (i) the disclosure of patent information by branded drugmakers, (ii) patent certificate and notice, (iii) stay of ANDA approval for a period of 30 months and (iv) 180 days of market exclusivity for the first qualified ANDA filer.
Within the legal framework of patent linkage, the patent certificate and notice and stay of ANDA approval mechanisms provide branded drugmakers an opportunity to resolve patent disputes before generic drugs are launched on the market. At the same time, generic drugmakers benefit from the identification of relevant patents by the disclosure of patent information. In addition, the first ANDA filer which successfully challenges the validity of a pharmaceutical patent enjoys 180 days of market exclusivity.
Table 2: Main mechanisms of patent linkage
In Favor of Branded Drugmakers (NDA Holders) |
In Favor of Generic Drugmaker (ANDA Holders) |
To resolve patent disputes before generic drugs reach the market |
To identify relevant patents covering the reference drug |
- patent certificate and notice
|
- disclosure of patent information by branded drugmakers
|
- 30-month stay of ANDA approval
|
- 180-day market exclusivity for the first qualified ANDA filer
|
Implementation of Patent Linkage in Other Countries
Under the influence of the FTAs entered into with the USA, some countries have established patent linkage systems or similar mechanisms in a variety of ways, after considering the domestic legal system and consulting with the pharmaceutical industry. Within the legal framework of patent linkage in South Korea, for example, the period of stay of ANDA approval is 9 months and the first ANDA filer enjoys 9 months market exclusivity. The patent linkage in Canada, meanwhile, does not include the mechanism of market exclusivity for the first ANDA filer, even though this is essential for the domestic generic drug industry, as it rewards the ANDA filer for undertaking the risk of being sued for patent infringement in applying for market approval.
In contrast to the patent linkage approach of the USA, Australia and Singapore have established “soft linkage,” which omits the disclosure of patent information and market exclusivity for the first ANDA filer mechanisms. Nevertheless, under the soft linkage system, ANDA filers need to follow the patent certificate step and give the branded drugmaker notice if the ANDA filer plans to market the generic drug before the expiry of the patent. Instead of a stay of ANDA approval, the soft linkage applies a preliminary injunction to prevent the allegedly infringing pharmaceuticals from being launched on the market.
The Current Ineffective Drug Approval-Patent Linkage System in Taiwan
According to Article 40ter (3) of Taiwan’s Pharmaceutical Affairs Act, three years after attaining market approval for a New Chemical Entity (NCE) drug, generic drugmakers are allowed to file for an ANDA and can reference the undisclosed data of the reference drug. On the day following the day on which the five year data exclusivity term expires, generic drugmakers can then begin to market their products. The above-mentioned regulations apply only to NCE drugs. That is to say, approval of generic drugs can be filed, granted and launched on the market at any time, if the reference drug is a “505(b)(ii) new drug”, including new combinations, new routes of administration and new dosages of drugs.
When it comes to the early resolution of patent disputes, the Pharmaceutical Affairs Act adopts an ineffective IPR-declaration mechanism. When applying for market approval, the ANDA filer is obligated to declare that the generic drug does not infringe any IPRs of the reference drug. However, the TFDA may approve it for the market regardless of whether the declaration is correct or false, and whether or not the reference drug is protected by patents. Nor is there any legal effect if the ANDA filer submits an incorrect or false declaration.
Even though the ANDA filer is obliged to submit an IPR-declaration, they are not required to give the branded drugmaker notice. For this reason, only after the generic drug launches on market, will the branded drugmaker become aware and be in a position to file a lawsuit. The premature launch of generic drugs usually causes commercial harm that is difficult to compensate after the fact. The remedy provided by preliminary injunctions to prevent the infringing pharmaceuticals from being launched on the market is not really feasible either. Unlike the “soft linkage” in Australia and Singapore, Taiwan follows the double-track system of civil law and administrative law. A preliminary injunction ordered by a civil court, in accordance with the civil procedure law, is limited to civil action and has no influence on the grant of market approval, which is an administrative matter. In other words, a preliminary injunction ordered by a civil court neither prevents the TFDA from granting market approval nor does it prevent the National Health Insurance Administration (NHIA) from including the infringing pharmaceuticals on reimbursement lists.
The current IPR-declaration mechanism has been criticized by branded drugmakers who assert that Taiwan should establish a proper system to effectively prevent the marketing of patent-infringing generic drugs. After consideration of the domestic legal system and the generics industry, the draft amendment of the Pharmaceutical Affairs Act introducing the legal framework of patent linkage was proposed by the TFDA and subsequently approved by the Executive Yuan in August 2016.
Looking Forward
Since the 1980s Taiwan has designated biotechnology and pharmaceuticals as key development areas, and has striven to establish strong domestic industries. Following the Action Plan for Strengthening the Biotechnology Industry in 1995, the government introduced the Biotech and New Pharmaceutical Development Statute in 2007 and implemented the Taiwan Diamond Action Plan for Biotech Takeoff in 2009, aimed at upstreaming R&D and increasing the availability of venture capital funds.
The patentability of chemical and pharmaceutical products and patent term extensions were introduced into the Patent Act in 1986 and 1994 respectively. Observing the status of pharmaceutical and biotechnology patents in Taiwan, it is clear that there is a growing trend in the numbers of domestic patent assignees, according to TIPO’s annual reports from 2003 to 2016.
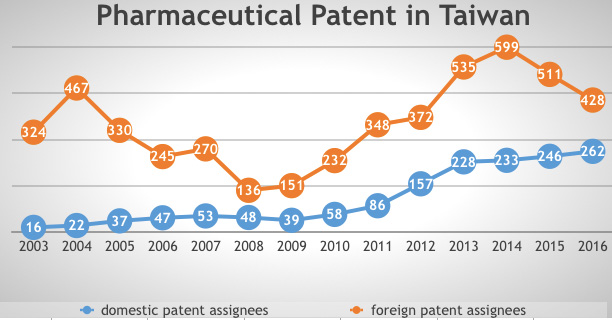
Figure 2: Pharmaceutical patent in Taiwan (Source: TIPO’s annual report)
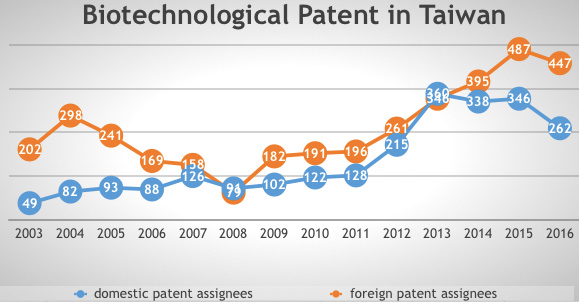
Figure 3: Biotechnological patents in Taiwan (Source: TIPO’s annual report)
The growing trend suggests that the domestic industry has become aware of the importance of intellectual property protection and is attempting to accumulate patents in these sectors. It also reveals that the domestic biopharmaceutical industry is developing somewhat sluggishly but steadily.
To keep up with global development trends, in 2016 the Executive Yuan approved the Taiwan Bioeconomy Industry Development Program and established the Center of Biomedical Industry Innovation Program in January of this year. The author advocates that policy on industry promotion should also turn attention to the intellectual property issues involved, as well as funding provision and the allocation of governmental resources. To commercialize biopharmaceutical products and increase their added value, intellectual property is indispensable. It plays a crucial role in innovation and provides incentive for sustaining long-term investment.
The protection of pharmaceutical inventions in the Patent Act, including patentability and patent term extension, is in compliance with the policy goal of encouraging innovation. However, intellectual property has little value without enforcement. The current drug approval-patent linkage and IPR-declaration mechanism enshrined in the Pharmaceutical Affairs Act are ineffective. The author supports the draft amendment of the Pharmaceutical Affairs Act and asserts that the proposed patent linkage is essential to achieve the action plan of promoting the further development and increasing the competitiveness of the domestic biopharmaceutical industry.
In addition to patent linkage, another resolution mechanism for avoiding pharmaceutical patent infringement is an approach wherein a long period of data exclusivity is granted, as adopted by European countries and Japan. In Europe, for example, branded drugmakers enjoy at least 10-years protection of data exclusivity, during which time the data from their pre-clinical and clinical trials cannot be accessed or referenced by generic drugmakers. Specifically, generic drugs may be marketed only after the expiry of the 10-year data exclusivity term. Under these circumstances, most patents covering the drugs in question will have expired. The 10-year data exclusivity approach drastically reduces the risk of patent infringement resulting from the marketing of generic drugs.
 |
|
| Author: |
Su-hua Lee |
| Current Post: |
Associate Professor, College of Law, National Taiwan University |
| Education: |
PhD, College of Law, National Taiwan University
L.L.M., Faculty of Law and Economics, University of Bonn, Germany
L.L.B., College of Law, National Taipei University |
| Experience: |
Assistant Professor, College of Law, National Taipei University
Assistant Professor, Institute of Law for Science & Technology, National Tsing Hua University |
| Research Fields: |
- Intellectual Property Law
- IP in the Pharma Industry
- Competition Law
- Civil Law
|
|
|
|
| Facebook |
|
Follow the IP Observer on our FB Page |
|
|
|
|
|
|

